
Answer to CPC #2 (Tuesday, October 22, 2002)
Case discussion: Pamela A. Lipsett, MD
This patient reportedly had a very satisfactory course for the first three years after orthotopic hepatic transplantation done for cirrhosis secondary to chronic Hepatitis C infection, the most common identified disease for hepatic transplantation today. We are not told about his transplant medications at the time of admission. He presented with a one week history of fever, chills, malaise, productive cough, and a reported Pseudomonas urinary track infection having had appropriate antibiotic treatment. In addition, he presented with hyperbilirubinemia and evidence of acute and chronic rejection on hepatic biopsy at the time of admission. After treatment with steroids, he developed progressive acute on chronic renal failure, and coagulopathy. MRSA bacteremia followed. Shortly thereafter, he developed a pericardial effusion requiring treatment, cardiac dysrhythmias, declining mental status, hypoxemia and pancytopenia. Chest X-ray demonstrated alveolar infiltrates in both upper lobes, focal ill-defined alveolar infiltrates in the right lower lung, and atelectasis in the left lower lobe. MRI scan of the brain showed multiple non-enhancing acute infarctions as his mental status continued to worsen. His medical history is of interest for diabetes, nephrolithiasis, and interestingly, histoplasmosis, on sigmoid biopsy of colonic polyps in 2000.
Simply put this immunosuppressed
patient developed a proven urinary
track infection, concurrent infection
and hepatic graft rejection and
failure, followed by progressive
multisystem organ failure and death.
We must now discuss the potential
explanations for his clinical course.
Three general categories of diseases
causing multisystem organ failure
must be considered 1) infection
2) inflammation/rejection and 3)
unusual neoplastic syndromes.
Patients with solid organ transplantation are at increased risk for certain neoplasms, especially lymphproliferative diseases. Epstein-Barr virus (EBV) infects B lymphocytes in up to 90% of the population. EBV infected cells B cells many undergo many rounds of proliferation with each cell harboring the virus. Though we are not told about his particular baseline immunosuppressive medications, T cell immunosuppressives (OKT3) allow proliferation of the B cell population. If the immune system has successfully eliminated the EBV initial infection, reactivation does not occur except in transplant and other severely immunosuppressed patients. At least 50% of solid-organ transplant patients harbor the virus in the oropharynx, and are at risk of the EBV-associated lymphoproliferative disorder, with at least 5% of all patients developing the disease. Additional neoplasms with widespread system involvement as seen in this case include other lymphomas, Kaposi's sarcoma, metastatic solid tumor and leukemia. Some features of his current presentation, bilateral pulmonary infiltrates, and multiorgan involvement could be caused by this disease, but the predominant apical nature and the CT scan of the head are not typical. EBV associated disease can occur after transplantation and can be rapidly progressive; typically the onset would be soon after transplantation. I will therefore exclude this diagnosis from further consideration.
Noninfectious and non-neoplastic causes of fever, pulmonary infiltrates, and organ failure could include pancreatitis, chronic thromboembolism, collagen vascular disease, and amyloidosis to name just a few. Organ rejection, necrosis and superinfection could explain the general course of this patient. We are not told of any elevations in serum amylase, of any abdominal tenderness, or of any radiological evaluation of his abdomen. The head CT findings would then have to result from a second separate process. Chronic thromboembolism, collagen vascular disease, and amyloidosis are general systemic diseases that could mimic some of his findings but are unlikely to be the cause of his multisystem failure in light of his known diagnoses.
I will focus the remaining discussion on infectious processes that could explain this patient's clinical picture. The risk of infections in transplant patients can generally be thought of in two components: the intensity of exposure to a potential pathogen and the net state of immunosuppression. Even minimal environmental exposure to a pathogen of low virulence can cause invasive infection in a patient with a maximal level of immunosupression. Epidemiological exposure occurs both in the community and in the hospital. Short-term community exposure includes exposure to respiratory viruses and to food borne pathogens. Community exposure also includes current and remote exposure to geographically restricted systemic mycoses (such as Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum), Mycobacterium tuberculosis, and Strongyloides sterocalis. Excessive environmental exposure can occur through contamination of air or potable water with pathogens such as aspergillus, legionella, or gram negative bacteria such as Pseudomonas aeurginosa. Outbreaks of VRE, MRSA, and C. difficile are well described.
The net state of immunosuppression is related to the dose duration, and temporal sequence of individual agents and the presence or absence of infection with immunomodulating viruses (cytomegalovirus (CMV), EBV, Hepatitis B (HBV) ), and HIV, as well as the residua of any technical complications of the transplant. We know that this patient has been immunosuppressed with his post-transplant medications, with HCV, and further with recent steroid boluses.
In considering which infectious agents are likely to be involved in a particular patient, time from the transplant surgery can generally categorize the likely pathogens. We can generally consider those infections which occur within 1 month of transplantation, within 2-6 months, and after 6 months. Early after transplantation, infection can be transmitted from the donor to the recipient, with unusual cases such as the recent case of transmission of the West Nile Virus. More typically, we are concerned about transmission of bacteria and fungi from the donor to the recipient. Infection of the donor organ can result in vascular infection, mycotic aneurysms, and catastrophic rupture. Much like non-immunocompromised hosts, more than 90% of infections in the first month after transplant are secondary to bacteria or fungi and are related to infections of the surgical wound, lungs, urinary tract, or catheters .
During the 2-6 month period following transplantation, additional infectious etiologies must be considered. The immunmodulating viruses CMV, EBV, HBV, and HCV begin to exert clinically significant effects. The combination of sustained immunosuppression and viral infection makes possible opportunistic infections such as P carinii, aspergillus, and L. monocytogenes even in the absence of an excessive environmental hazard.
This patient is more than six months after transplantation. Generally, in a patient who is doing well, immunosuppressive therapy is minimized and infections are those of the general community and are generally respiratory. Opportunistic infection is unusual unless environmental exposure is intense. At least 10% of patients have chronic or progressive infection with HBV, HCV, CMV, or EBV or possible papillomavirus. In another 5-10% of transplant recipients, recurrent or chronic rejection develops with greater exposure to steroids, which often results in chronic viral infections. These patients are those at greatest risk of opportunistic infections including P. carinii, L monocytogenes, N asteriodes, Cryptococcus neoformans and Aspergillus. We know this patient had an immunmodulating virus (HCV) and recent steroid boluses so I am concerned that he is at special risk for opportunistic infections, as well as common bacterial infections.
I will approach his possible disease course by examining specific organ dysfunction, time course and possible pathogens.
This patient entered JHH with a UTI and developed progressive renal failure, pericarditis and sepsis. Given his history of renal calculi early in the course of his hospitalization one would have had to obtain a renal ultrasound and rule out an obstructed infected kidney as a cause of both infection and renal failure. Certainly, pericarditis and rhythm disturbances could be related to the progression of renal failure. In addition, the calcineurin inhibitors both can be associated with renal insufficiency and have many P450 interactions. Thus while drug toxicity may have had a role in chronic renal insufficiency, I am doubtful that these agents caused his acute renal failure. His chronic renal failure could also have been related to his diabetes. His acute renal failure was most likely associated with progressive hepatic failure and sepsis.
His progressive hepatic insufficiency was secondary to both recurrent HCV and mild to moderate rejection. Recurrence of HCV occurs in virtually all patients after liver transplantation with 10-20 fold levels of viremia. The natural history of this hepatitis is characterized by progression to cirrhosis in 6-23% at a median of 3 to 4 years post-transplantation. The development of cirrhosis is associated with reduced graft and patient survival. Several studies have demonstrated that degree of immunosuppression defined as the number of methylprednisilone boluses, use of antilymphocyte globulin, and total cumulative dose of corticosteriods is strongly associated with both a greater incidence of recurrent hepatitis C and a more aggressive course. Many centers have chosen to apply immunosuppressive regimens with rapid steroid withdrawal. However, this recent change in therapy has not been proven effective and recent studies suggest that disease progression has increased in recent years. This patient had both HCV recurrence and rejection and was treated with repeated high dose steroid therapy. We must then consider whether his subsequent prolonged hospital course is related to another acquired infection, reactivation of a known infection, or simply progression of his underlying hepatic and consequently renal diseases.
While in the intensive care unit, he developed S. aureus bacteremia. We are not told whether it was associated with a vascular catheter. S. aureus is common nosocomial pathogen and is particularly associated with vascular catheters, endovascular, and pneumonic processes. We are told that he had a single positive blood culture for this pathogen. While S. aureus has a propensity for metastatic infection and certainly could have easily been involved in myocardial or valvular infection with subsequent embolism to the brain, this explanation is unlikely with a single blood culture, and the character of the brain lesions is not typical for an embolic infectious source. Specifically we would expect a lesion that would have some aspect of rim enhancement. In addition, we are not told of a valvular lesion on echocardiography. However, the study was most likely a transthoracic study and a transesophageal study would have been a more definitive study to answer this question. The presence of the bilateral upper lobes alveolar infiltrates, focal ill defined infiltrates could be caused by S.aureus. However, S. aureus grows easily in culture and the pattern of infection is more typically lobar only rarely miliary in origin. A negative bronchoalveolar lavage makes S. aureus pulmonary infection with septic shock and progressive end organ failure with metastatic lesions to the brain unlikely.
We must now consider the remaining symptoms, signs, laboratory, and radiological information. This patient had bilateral alveolar infiltrates greatest in the upper lobes, which progressed rapidly since admission. We must remember however that at his original presentation he had a cough, fever, and malaise. He also had declining mental status with multiple infarcts not restricted to a specific intracranial distribution indicating most likely a hematogenous source of emboli or necrotizing infection. We must consider that he arrived with a mild headache but without visual disturbance. We will want to consider diseases that combine both of these processes. Though his pericardial disease could be explained by progressive renal failure and uremia, we will want to additionally consider diseases that also involve pericardium and possibly myocardium. We will want to consider diseases that are associated with bone marrow suppression or involvement, and those that are more likely to occur and rapidly progress in patients with immuno and marrow suppression.
Apparently trivial complaints such as a persistent dry cough may prove to be an early sign of impending pneumonia from Aspergillus spp., respiratory syncytial virus, or influenza virus. Thoracic computed tomographic scans are more sensitive in detecting pulmonary infiltrates compatible with aspergillosis than are plain chest radiographs. We will come back to this diagnosis. Bronchoalveolar lavage specimens from patients with pulmonary infiltrates should have a battery of tests that usually includes smears for Pneumocystis carinii, the acid-fast bacilli and Nocardia spp., bacteria, and molds, as well as culture for fungi and bacteria, including Legionella spp., Mycobacterium spp., Nocardia spp., and respiratory viruses (influenza and parainfluenza viruses, adenovirus, respiratory syncytial virus (RSV), and cytomegalovirus). Rapid assays by enzyme-linked immunosorbent assay, direct fluorescent antibody, or dot blot are also available for some of these viruses. Nasopharyngeal swabs can detect respiratory syncytial virus and can be tested by one of the rapid methods. While RSV could explain the pulmonary infiltrates, there is no report of any coryza, or nasal congestion. In addition outbreaks of RSV typically occur in this area from November to April and not in May as in this case. Parvovirus can explain his bone marrow suppression but is unlikely to explain his remaining symptoms complex. Cytomegalovirus , either primary or reactivation disease is common in transplant patients but tends to occur in the 2-6 month period. However, reactivation can occur with episodes of immunosuppression. Pneumonitits, bone marrow suppression, retinitis, esophagitis, hepatitis, myopericarditis, meningitis and encephalitis can all be present with CMV disease. While most of his clinical picture could be explained by CMV reactivation, and he is at risk, the timing of the disease is unusual, and the CT findings not typical. We are not told of a positive antigen or other confirmatory invasive biopsy.
A number of organisms are transmitted through the air from the physical environment, particularly fungi such as Aspergillus, Coccidioides, Histoplasma, and Cryptococcus. Aspergillus, cryptococcal, and nocardial infections are seen in all geographic regions, but post-transplantation coccidioidomycosis is uniquely a problem of certain endemic regions, and this is not typically seen in Baltimore so I will not consider it further.
The major presenting symptoms of P. carinii pneumonia in the compromised host are shortness of breath, fever, and a nonproductive cough. On physical examination, tachypnea and tachycardia are found in acutely ill patients. The chest radiograph classically exhibits bilateral diffuse infiltrates extending from the perihilar region Atypical manifestations have ranged from normal films to unilateral infiltrates, nodules, cavities, pneumatoceles, lymphadenopathy, and effusion. Extrapulmponary P. Carinii has been occasionally reported, especially among HIV patients. Among the focal manifestations of extrapulmonary pneumocystosis are a rapidly enlarging thyroid mass, pancytopenia from bone marrow necrosis, retinal cotton wool spots, polypoid lesions in the external auditory canal, pleural effusion, numerous hypodense lesions in the spleen on computed tomography and punctate calcifications in the spleen, liver, adrenal, or kidney. Because lesions outside the lung are unusual we will exclude this diagnosis from further consideration.
Nocardiosis should always be considered in the differential diagnosis of indolent pulmonary disease, particularly in the setting of cellular immune compromise, along with other actinomycetes (e.g., Mycobacterium, Actinomyces spp.) and eumycetes (e.g., Cryptococcus neoformans, Aspergillus spp.). Pulmonary disease is the predominant clinical finding of nocardia (more than 40% of reported cases) with almost 90% of such cases caused by members of the N. asteroides complex. Clinical manifestations of established infection include endobronchial inflammatory masses, pneumonia, lung abscess, and cavitary disease with contiguous extension to surface and deep structures, including effusion and empyema. Radiological manifestations include irregular nodules (usually cavitating when large), reticulonodular or diffuse pneumonic infiltrates, and pleural effusions. Pulmonary nocardiosis may occur as alveolar infiltrates rather than cavitary disease in immunosuppressed patients. Clues to a nocardial etiology include spread to contiguous structures, especially with soft tissue swelling or external fistulas, and to the CNS. This disease frequently progresses over months to years. Disseminated infection is characterized by widespread abscess formation. The most commonly reported sites include the CNS and eyes (particularly the retina), skin and subcutaneous tissues, kidneys, joints, bone, and heart. Typically the CT will show a mass lesion often confused with a neoplasm. The absence of skin lesions, the lack of information about the eye examination and the lack of a typical CT scan will remove this cause from additional consideration.
The course of toxoplasmosis in almost all immunocompetent individuals is relatively benign, but it is a serious and often life-threatening disease in immunodeficient patients. Toxoplasmosis in these patients may be due to either newly acquired or reactivated latent infection. In immunosuppressed patients, 76% have CNS, 58% myocardial, and 23% pulmonary involvement. Toxoplasmosis with multiorgan involvement manifesting with acute respiratory failure and hemodynamic abnormalities similar to septic shock has been reported. The characteristic presentation usually has a subacute onset with focal neurologic abnormalities in 58 to 89% of patients. However, in 15 to 25% of cases, the clinical presentation may be more abrupt, with seizures or cerebral hemorrhage. The diagnosis may be made by demonstration of the parasite in BAL fluid. Illness may be clinically indistinguishable from Pneumocystis carinii pneumonia (PCP). Extrapulmonary disease may be present in about 50% of cases with toxoplasmic pneumonitis. Though a CT scan may be normal, typical a classic ring enhancing lesion is seen. The BAL in this case did not reveal this pathogen making it unlikely. Moreover, exposure to a source of this parasite, namely a cat is not described, so we will leave this diagnosis as well.
The onset of CNS cryptococcosis may be acute or insidious. Acute manifestations are more common in those receiving corticosteroids. Symptoms may be referable to the CNS, although they may be mild and nonspecific and include headache, irritability, clumsiness, and obtundation. Most patients have minimal or no nuchal rigidity. Papilledema is noted in up to one third of cases and cranial nerve palsies in about one fifth. Visual loss may be total. Pulmonary cryptococcosis may be asymptomatic or may cause the production of only scant, sometimes blood-streaked sputum. Patients may present with cough and dyspnea. Single or multiple skin lesions may be found in 5 to 10% of patients. There is no report of skin lesions and no report of a potential exposure to bats or to pigeons. CT or MRI findings may be normal or reveal diffuse atrophy, cerebral edema, hydrocephalus, or focal mass lesions. Multiple nonenhancing lesions may be present, most often in the basal ganglia and thalamus, but sometimes at other sites, including infratentorial areas. On T2 -weighted MRI, nonenhancing parenchymal cryptococcomas may also be associated with nonenhancing, hyperintense, dilated perivascular Virchow-Robin spaces. CT or MRI may demonstrate diffuse atrophy or cerebral edema, as revealed by focal homogeneous or doughnut-shaped, contrast-enhanced areas with or without surrounding circumferential areas of decreased density, even in asymptomatic patients. Cryptococcal masses must be distinguished from other causes of such intracranial mass lesions, including pyogenic, nocardial, or Aspergillus-associated abscesses, tuberculosis, toxoplasmosis, hemorrhage, lymphoma, or other neoplasms. MRI, particularly with gadopentetate dimeglumine enhancement, is more sensitive than CT scanning. Multiple small enhancing subarachnoid or parenchymal nodules may be present. Various other patterns are seen less often, including segmental pneumonia, thick-walled single cavities, lymphadenopathy, pleural effusion, and generalized miliary disease. The clinical findings in such patients are often indistinguishable from those of patients with acute pneumonia caused by Pneumocystis carinii, Mycobacterium tuberculous, Histoplasma capsulatum, or other organisms. Bronchoscopy with washings and brushings is usually diagnostic and we are not told of this on the BAL, this we will exclude this diagnosis as well.
With an appropriate remote history or with environmental exposure, development of primary or reactivation of Mycobacterium tuberculosis could explain this patient's diffuse system involvement and bone marrow suppression. Tuberculous pericarditis is most often caused by extension from a contiguous focus of infection, usually mediastinal or hilar nodes, but also the lung, spine, or sternum. Less commonly, it occurs during miliary tuberculosis. Tuberculous pericardial fluid demonstrates many of the characteristics of tuberculous pleural fluid, with acid-fast smears being rarely positive and cultures being positive in approximately 50% of cases. Postprimary pulmonary tuberculosis in adults is usually asymmetric and characterized by caseation, cavity formation, and fibrosis. It begins as a patch of pneumonitis in the subapical posterior aspect of an upper lobe, usually just below the clavicle or first rib. Bronchogenic spread may establish foci of infection in the lower lobe and anterior portions of the upper lobe, producing a polymorphous mottling on chest roentgenogram. CNS involvement from Mycobacterium usually is a mennigeal rather than a necrotizing lesion as seen in this case. Thus on the basis of no previous history, no known exposure, and an apparent negative BAL I will also exclude this diagnosis.
Listeria may arise from contaminated food sources, but a source is rarely identified in the sporadic cases of meningitis that are seen in the transplantation population. Many patients with listerial bacteremia or CNS infection give a history of antecedent gastrointestinal symptoms including diarrhea, nausea, and vomiting, often accompanied by fever. Listerial endocarditis accounts for about 7.5% of adult listerial infections and can produce both native valve and prosthetic valve disease, and has a high rate of septic complications and a mortality of 48%. Though pleuropulmonary infection can occur it is not a hall mark feature of the disease. In addition, the typical CNS presentation of meningitis involve a fluctuating mental status, seizures and movement disorders. Blood cultures are usually positive. I will thus exclude this from my diagnosis.
This patient had a history of Histoplasma found on a gastrointestinal biopsy many months ago. We do not know about the type, duration or success of his therapy. Thus we need to consider whether either acute or subacute progressive histoplasmosis could account for this clinical picture. In acute progressive disseminated histoplasmosis (PDH), the onset is usually abrupt. Fever and malaise are the two most common manifestations, followed by weight loss, cough, and diarrhea. Physical findings include hepatosplenomegaly in nearly all patients, lymphadenopathy especially of the cervical chain in about 30%, and rales. Jaundice is observed in a minority, and oropharyngeal ulcers may be seen. Anemia, leukopenia and thrombocytopenia are frequently seen. Serum levels of the liver enzymes alanine aminotransferase and alkaline phosphatase may be elevated in a high proportion. Chest roentgenograms most often reveal a patchy pneumonitis with mediastinal and hilar node enlargement. Up to 20% of patients with PDH have central nervous system involvement. The more aggressive forms include encephalitis, acute meningitis, and encephalopathy in acute PDH. Histoplasmoma of the central nervous system and chronic meningitis are manifestations of a more indolent form of PDH. Chest roentgenograms typically demonstrate widely scattered nodular opacities or a diffuse reticular pattern.
Infrequently, patients exhibit a sepsis-like syndrome characterized by disseminated intravascular coagulation, encephalopathy, acute respiratory distress syndrome, vascular collapse, and, subsequently, multiorgan failure. Subacute PDH is distinguished from the acute form primarily by the more prolonged nature of the symptoms before patients seek medical attention. Physical findings include hepatosplenomegaly and oropharyngeal ulcers. One of the notable features of subacute PDH is the presence of focal lesions in various organ systems, including the gastrointestinal tract, endovascular structures, central nervous system, and adrenal glands. Aside from liver and spleen, the gastrointestinal tract is one of the organs most commonly affected in subacute PDH. Endocarditis and infection of other vascular structures may be manifestations of subacute PDH. On echocardiography, the lesions tend to be extensive and large vessel embolization can be the presenting symptom. Central nervous system infection involves all age groups and causes a number of manifestations including chronic meningitis, mass lesion, and cerebritis. Among these, chronic meningitis is the most frequent. Symptoms include headache, altered sensorium, and cranial nerve deficits. Associated physical findings consist of hepatosplenomegaly in about a third, lymphadenopathy, and mucocutaneous lesions.
Histoplasmoma causes a mass effect and may initially be mistaken for a malignancy or abscess on computed tomography because it exhibits ring enhancement with the administration of contrast medium. Histoplasmomas may be associated with meningitis but are often independently present. Though this patient was known to previously have Histoplasmosis and he has pulmonary infiltrates, lack of hepatospleenomegaly, and lack of mass enhancement make this diagnosis unlikely.
I believe the final clue to solving this case lies in the analysis of the CT and MRI scans of the brain. The characteristic CT appearance of brain abscess is that of a hypodense center with a peripheral uniform ring enhancement following the injection of contrast material; this is surrounded by a variable hypodense area of brain edema. Other CT findings include nodular enhancement and areas of low attenuation without enhancement, the latter of which is observed during the early cerebritis stage before abscess formation; as the abscess progresses, contrast enhancement is observed. Once the abscess becomes encapsulated in the later stages, contrast material no longer differentiates the lucent center and the CT appearance is similar to that of the early cerebritis stage. MRI is more sensitive than CT and, therefore, offers significant advantages in the early detection of cerebritis, cerebral edema with greater contrast between edema and adjacent brain, more conspicuous spread of inflammation into the ventricles and subarachnoid space, and earlier detection of satellite lesions. On T1 -weighted images, the abscess capsule often appears as a discrete rim that is isointense to mildly hyperintense. Contrast enhancement with the paramagnetic agent gadolinium diethylenetriaminepentaacetic acid provides the added advantage of clearly differentiating the central abscess, surrounding enhancing rim, and cerebral edema surrounding the abscess. On T1 -weighted images, enhancement of the abscess capsule occurs. On T2 -weighted images, the zone of edema that surrounds the abscess is one of marked high signal intensity; the capsule now appears as a well-defined hypointense rim at the margin of the abscess. It is important to note that therapy with corticosteroids can decrease enhancement with both CT and MRI and thus many of the previous diagnoses I have excluded on the basis of the CT lack of rim enhancement COULD be present but the CT and MRI modulated by the steroids. However, there remains a diagnosis which I believe fits well with this patients course.
Though fungi are infrequently recognized as a cause of pericarditis, Candida spp., Aspergillus spp., Cryptococcus neoformans, and other fungi can cause disseminated infection in severely debilitated and immunocompromised patients, especially those with prolonged neutropenia who are receiving multiple courses of antibiotics. Overt myocarditis is common in disseminated toxoplasmosis, and systemic aspergillosis and candidiasis may also involve the heart.
Since the patient was managed with fluconazole, we must consider fungal pathogens that are not treated with this agent. We have previously considered both Cryptococcus and Toxoplasmosis and will now consider Aspergillus further.
Most patients with invasive aspergillosis have pulmonary disease (80 to 90%). Invasive pulmonary aspergillosis (IPA) is manifested differently in various patient groups. Patients who are most immunocompromised have few symptoms initially and the progression rate is fast (acute IPA). The earliest symptoms are a dry cough and low-grade fever. Hypoxemia is usual in persons with bilateral diffuse IPA or in those with extensive consolidation. The appearance of invasive aspergillosis on plain chest radiographs is heterogeneous. Consolidation is common. Cavitation and pleural-based wedge-shaped lesions are the most distinctive features of invasive aspergillosis. Nodular shadows, with and without cavitation, thick- or thin-walled cavities and "alveolar" consolidation that coalesces over time to form small nodules and areas of consolidation are typical. Diffuse, usually lower lobe, fine shadowing is also seen. Pleural effusions are uncommon. High-quality CT scans of the chest can play a major role in early diagnosis. The most distinctive early lesions are one or more small nodules and small pleural-based lesions with straight edges and surrounding low attenuation (the "halo" sign), particularly in neutropenic patients. As IPA progresses, the nodules may cavitate (typically with neutrophil recovery) and reveal an "air-crescent" sign. Both the "halo" and "air-crescent" signs are highly distinctive for invasive fungal disease of the lung and are usually caused by Aspergillus, occasionally by other molds, and rarely by Pseudomonas aeruginosa. These lesions represent infarcted lung tissue full of hyphae that extend beyond the area of infarction. Bronchoscopy is essential if airway disease is possible but rarely yields Aspergillus on culture especially if the patient has peripheral, focal disease of the lungs. Though we are not told of a classic chest CT finding, the chest radiograph and negative BAL, along with his risk factor allow us to consider this diagnosis further.
Cerebral aspergillosis occurs in about 10 to 20% of cases of invasive aspergillosis, usually as the worst manifestation of disseminated disease. Patients with Aspergillus brain abscess most commonly manifest signs of a stroke syndrome (secondary to ischemia or intracerebral hemorrhage, or both) referable to the involved area of brain. Thus, I believe that his CT and MRI findings, his pulmonary disease, bone marrow involvement, and risk factors make disseminated Aspergillus infection the most likely diagnosis and cause of death. In the absence of microbiological or pathological material other very very unusual emerging pathogenic fungi would also be possible (ie Fusarium, Scedosporium, Scopulariopsis, etc).
Pamela A. Lipsett's Diagnosis:
- Recurrent hepatitis C infection post-transplantation with cirrhosis
- Acute on chronic renal failure secondary to acute tubular necrosis, chronic drug exposure, and diabetes
- Pericarditis, myocarditis, pulmonary, cerebral and bone marrow involvement with disseminated Aspergillus infection.
Autopsy Findings:
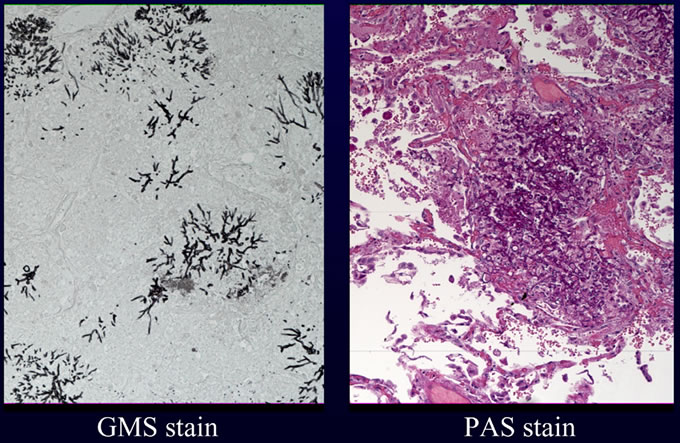
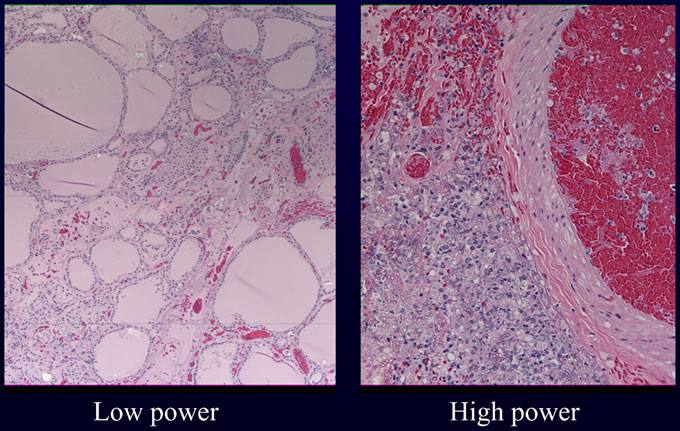
An autopsy excluding examination of the head was requested and performed as per the wishes of the patient's family. Internal examination revealed numerous lesions, most less than one centimeter, varying from tan nodules with hyperemic rims to hemorrhagic ulcers with necrotic centers. These lesions were present on numerous organs including the heart, lung, gastrointestinal tract, kidneys and thyroid. Histologic examination of these lesions revealed collections of septate fungal hyphae with acute dichotomous branching. The presence of fungi was highlighted with silver (GMS) and PAS stains. Examples of lesions involving heart (Figures 1A, 1B), lungs (Figures 2A, 2B) and thyroid (Figure 3) are illustrated below. These morphologic features, in addition to the angioinvasion in some of the sections, are strongly suggestive of disseminated infection with Aspergillus species. However, in the absence of confirmation by culture with demonstration of characteristic fruiting bodies, one cannot speciate this fungus. Other fungal pathogens with similar morphologies must be considered, including Fusarium species, and Pseudallescheria boydii. Clinical features favor the diagnosis of Aspergillosis. First, these other fungi are far less common human pathogens than are Aspergillus species. Second, the patient lacked a history of water exposure to suggest Pseudallescheria boydii. Thus, the presumptive diagnosis in this case is invasive Aspergillosis.
Figure
1A
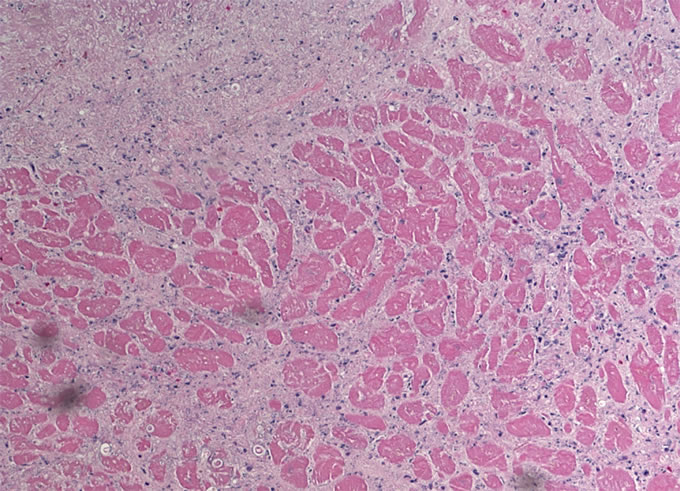
Figure 1B
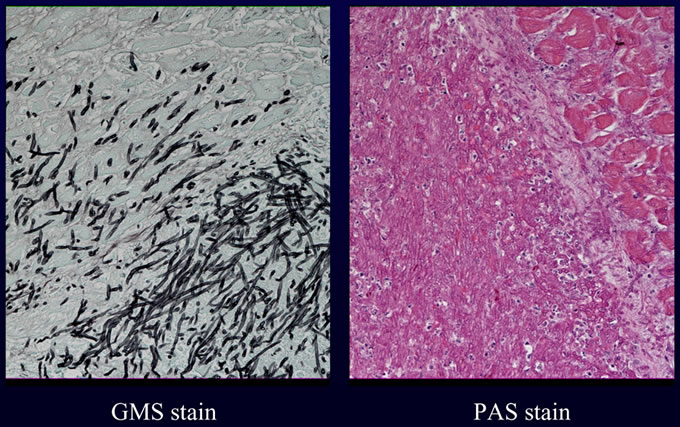
Figure
2A
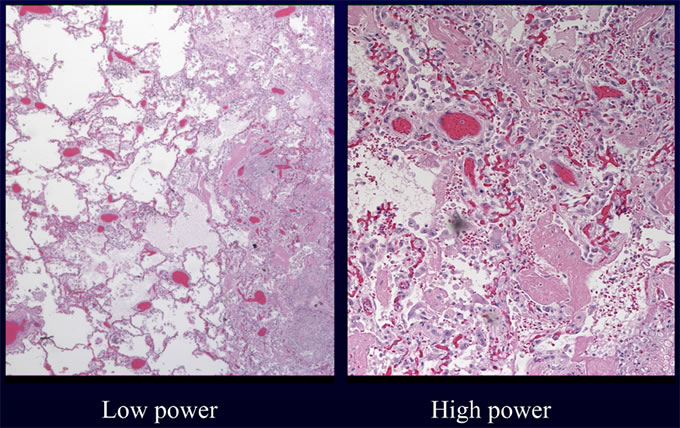
Figure
2B
Figure
3
References for Dr. Lipsett's Discussion:
1. Immunocompromised Host Society Consensus Conference on Epidemiology, Prevention, Diagnosis, and Management of Infections in Solid-Organ Transplant Patients. Clin Infect Dis. 2001 Jul 1;33 Suppl 1:S1-65
2: Simon DM, Levin S. Infectious complications of solid organ transplantations. Infect Dis Clin North Am. 2001 Jun;15(2):521-49
3: Patterson TF. Approaches to fungal diagnosis in transplantation. Transpl Infect Dis. 1999 Dec;1(4):262-72
4: Patterson JE. Epidemiology of fungal infections in solid organ transplant patients. Transpl Infect Dis. 1999 Dec;1(4):229-36
5: Hsieh WS, Lemas MV, Ambinder RF. The biology of Epstein-Barr virus in post-transplant lymphoproliferative disease. Transpl Infect Dis. 1999 Sep;1(3):204-12.
6: Snydman DR. Infection in solid organ transplantation. Transpl Infect Dis. 1999 Mar;1(1):21-8.
7: Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001 Jun;3(2):70-8
8: Snydman DR. Epidemiology of infections after solid-organ transplantation. Clin Infect Dis. 2001 Jul 1;33 Suppl 1:S5-8.
9: Rubin RH, Schaffner A, Speich R. Introduction to the Immunocompromised Host Society consensus conference on epidemiology, prevention, diagnosis, and management of infections in solid-organ transplant patients. Clin Infect Dis. 2001 Jul 1;33 Suppl 1:S1-4.
10: Sampathkumar P, Paya CV. Fusarium infection after solid-organ transplantation. Clin Infect Dis. 2001 Apr 15;32(8):1237-40
11: Dictar MO, Maiolo E, Alexander B, Jacob N, Veron MT. Mycoses in the transplanted patient. Med Mycol. 2000;38 Suppl 1:251-8.
12:
Dupont B, Richardson M, Verweij
PE, Meis JF. Invasive aspergillosis.
Med Mycol. 2000;38 Suppl 1:215-24.
13: Cainelli F, Vento S. Infections
and solid organ transplant rejection:
a cause-and-effect relationship?
Lancet Infect Dis. 2002 Sep;2(9):539-49
14: Maschke M, Kastrup O, Diener HC. CNS manifestations of cytomegalovirus infections: diagnosis and treatment. CNS Drugs. 2002;16(5):303-15
15: Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation. Clin Infect Dis. 2001 Nov 1;33(9):1536-44
16: Ghobrial RM. Retransplantation for recurrent hepatitis C. Liver Transpl. 2002 Oct;8(10 Suppl 1):S38-43
17: Everson GT. Impact of immunosuppressive therapy on recurrence of hepatitis C. Liver Transpl. 2002 Oct;8(10 Suppl 1):S19-27.
18: Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002 Oct;8(10 Suppl 1):S14-8
19: Rakela J, Vargas HE. Hepatitis C: Magnitude of the problem. Liver Transpl. 2002 Oct;8(10 Suppl 1):S3-6.
20: Charlton M. Hepatitis C infection in liver transplantation. Am J Transplant. 2001 Sep;1(3):197-203.
21: Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002 Jun;121(6):1988-99.
22: Tolkoff-Rubin NE, Rubin RH. Viral infections in organ transplantation. Transplant Proc. 1998 Aug;30(5):2060-3
23: Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998 Jun 11;338(24):1741-51.
Textbook
disease reviews (substantial facts
and text reproduced from this textbook):
Mandell: Principles and Practice
of Infectious Diseases, 5th ed.,
Copyright (c) 2000 Churchill Livingstone,
Inc