
CPC #7: Answer
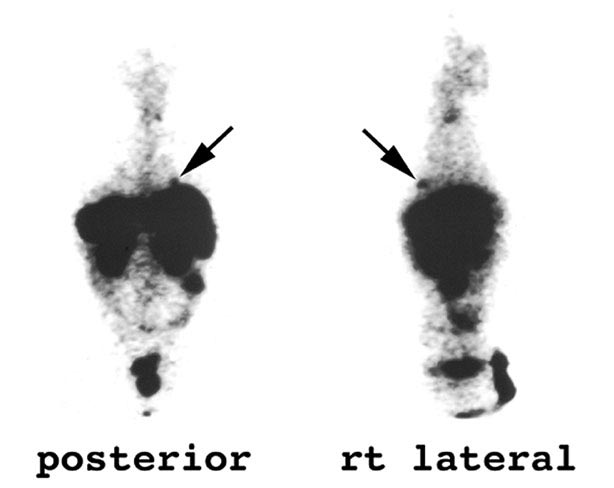
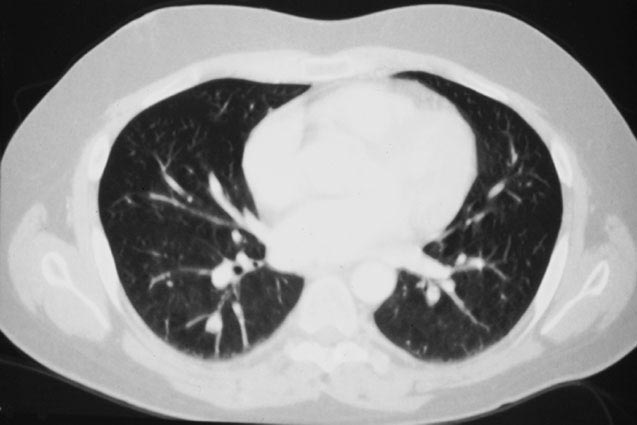
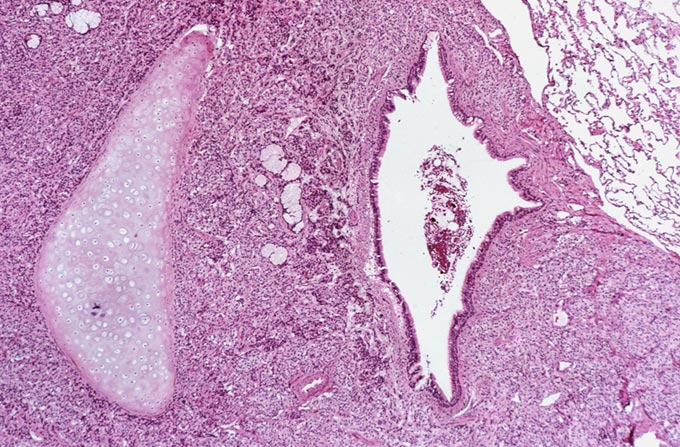
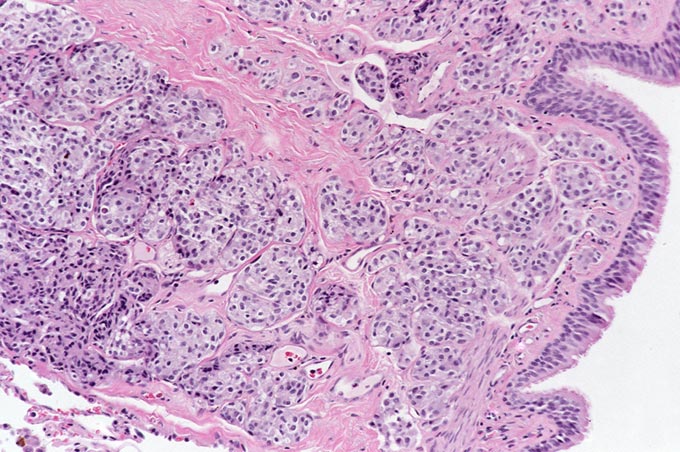
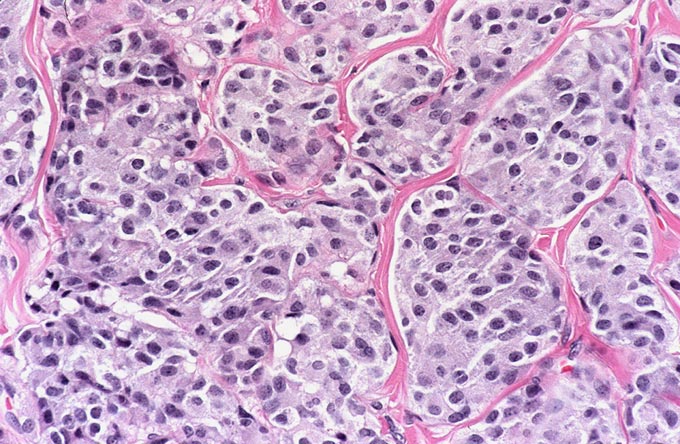
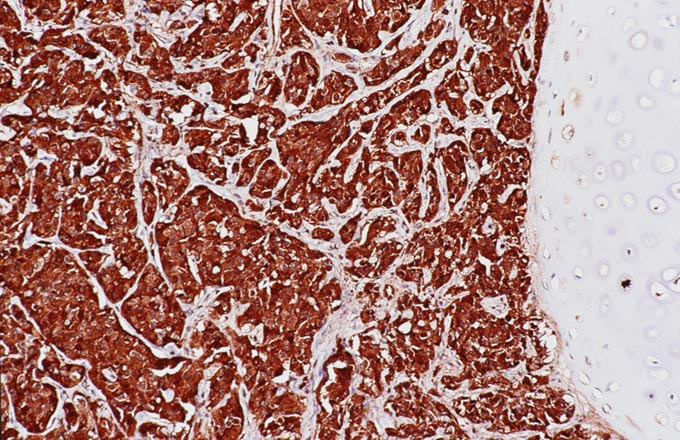
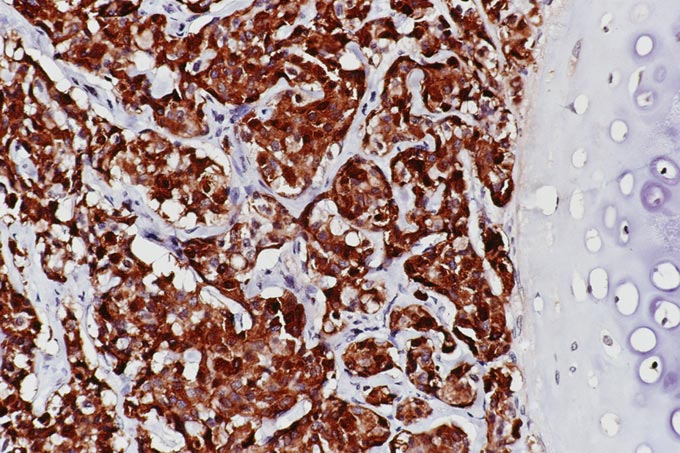
The patient underwent an octreotide scan, which utilizes a radioactive analog to somatostatin. The rationale for this is that neuroendocrine tumors throughout the body are characterized by somatostatin receptors, and imaging with this molecule can localize otherwise clinically occult tumors that have a proclivity to produce ACTH. In this patient, a 1-cm. nodule was identified in the right lower lobe, possibly obscured on the chest x-ray by the dome of the diaphragm (Figure 1). This peribronchial nodule was evident on a subsequently performed CT scan of the lung (Figure 2). The patient then underwent right lobectomy, which revealed a 1 cm, peribronchial circumscribed fleshy mass. Microscopic examination revealed a nested neoplasm composed of epithelioid cells having fine “salt and pepper” chromatin and granular cytoplasm (Figure 3, 4, 5). Mitoses and necrosis were not evident. The tumor was immunoreactive for cytokeratin and chromogranin (Figure 6), which confirmed the diagnosis of carcinoid tumor. Based upon the clinical suspicion, the tumor was studied for expression of pituitary hormones. The tumor was non-reactive for prolactin and human growth hormone, but intensely immunoreactive with antibodies to ACTH (Figure 7). Hence, the diagnosis was that of a pulmonary carcinoid tumor ectopically producing ACTH, leading to Cushing’s syndrome.
This case demonstrates the challenges associated with the work-up of Cushing’s syndrome, defined as signs and symptoms resulting from hypercortisolism regardless of the etiology. Five main categories of lesion are in the differential diagnosis of Cushing’s syndrome. Iatrogenic Cushing’s syndrome is a frequent sequella of treatment with corticosteroids, but is excluded in the current case on the basis of clinical history. The most common cause of endogenous Cushing syndrome would be Cushing’s disease resulting from an ACTH producing pituitary adenoma. Cushing’s disease comprises 70% of all Cushing’s syndromes, and is high in the differential diagnosis in this patient, since the dexamethasone suppression tests were most consistent with this diagnosis, 70% of pituitary adenomas are non-visible on MRI scans and the surgical cure rate for this disorder at our institution is approximately 50%. In this case, the tumor may have been small and missed on the initial surgical procedure. ATCH-independent causes of Cushing’s are related to adrenal pathology and include benign adenoma, adrenal cortical carcinoma, bilateral nodular adrenal cortical hyperplasia, and primary pigmented nodular adrenal cortical hyperplasia; these are excluded by the high serum ACTH level. Ectopic production of CRH or ACTH make up the final two categories of lesion; these are most frequently produced by neuroendocrine neoplasms.
The best screening test for Cushing’s syndrome is the 24-hour urine free cortisol study. A urine free cortisol of greater than 200 micrograms (4 times the upper limit of normal) establishes the diagnosis of Cushing’s syndrome. Less dramatic elevations of urine free cortisol (between 50 and 200 micrograms) warrant analysis by a low dose dexamethasone suppression test. It is known that such slight elevations in urine free cortisol may result from “pseudo-Cushing’s syndrome,” which results from a variety of disorders including depression, anxiety disorder, alcoholism and obesity. The low dose dexamethasone suppression test is done by giving one half of a milligram of dexamethasone four times a day over two days. A 24-hour urine free cortisol of greater than 5 micrograms in this setting establishes the diagnosis of Cushing’s syndrome. However, it is important to remember that certain drugs (such as dilantin and tegritol) can induce dexamethasone clearance and render this test uninterpretable.
The next step is to determine the etiology of the Cushing’s syndrome. This is done using a high dose dexamethasone suppression test, which entails administering 2 milligrams of dexamethasone four times a day for a period of two days. The principle of this assay is that pituitary dependent ACTH production is reduced by high dose dexamethasone, while ectopic ACTH production is not suppressed by glucocorticoids. Hence, greater than 50% suppression supports the diagnosis of Cushing’s disease, while greater than 90% suppression virtually makes this diagnosis certain. Less than 50% suppression suggests either adrenal or ectopic hormone production as the etiology. These latter two are easily distinguished by the elevated ACTH in the ectopic setting, whereas ACTH is depressed in the setting of adrenal-driven Cushing’s syndrome
In this case, the high ACTH level excludes adrenal origin. The results of the dexamethasone suppression tests are equivocal. The urine free cortisol was suppressed 89% (which favors pituitary origin), while serum cortisol was suppressed only 39% (which suggests ectopic origin). The best study to perform in this case would have been inferior petrosal sinus sampling, which could help localize the lesion to the pituitary. In the absence of this study, the value of the octreotide scan in defining ectopic ACTH producing tumors is self-evident. However, there is a significant false positive and false negative rate associated with this imaging procedure.